CONDITIONS TREATeD / MULTIPLE SCLEROSIS
Regenerate. Repair. Restore.
Stem Cells Therapy for Sclerosis (MS) and diseases of the CNS


Multiple sclerosis affects the central nervous system.
The WORLD HEALTH STEM CELLS recommends the use of mesenchymal stem cells, derived, cultured, and expanded from umbilical cord tissue, for the treatment of neurological diseases including multiple sclerosis.
The treatment could help improve the symptoms of MS:
- Visual disturbances
- Loss of balance
- Poor coordination
- Slurred speech
- Tremors
- Numbness
- Extreme fatigue
- Problems with memory and concentration Paralysis
- Blindness
- Bladder and bowel problems
- Sexual dysfunction
- Muscle spasticity
- Sensitivity to heat
- Emotional disturbances
What do we know about MS?
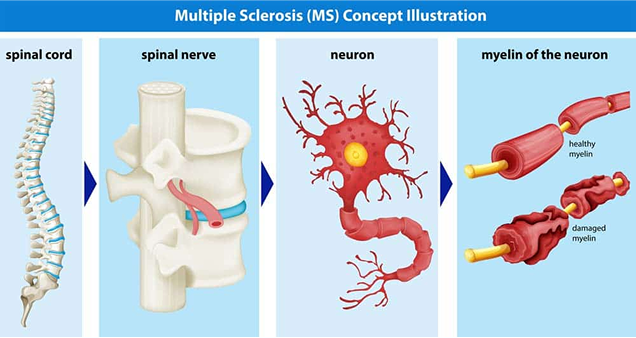
Multiple Sclerosis (MS) is a group of immune-mediated chronic disorders in which the immune system mistakenly attacks healthy tissue in the central nervous system.
MS is characterized by recurrent episodes of focal inflammatory demyelination resulting in neurological symptoms.
MS affects over 2.5 million people globally, and while it can affect anyone at any age, most people diagnosed are between the ages of 20 and 50.
Axons of the brain and spinal cord are nerve fibers that transmits information to different neurons, muscles, and glands in the body.
Multiple sclerosis is diagnosed when the immune system attacks and damages the protective myelin sheath, impacting the central nervous system’s ability to communicate with the rest of the body.
Eventually, the nerves begin to degenerate and might become permanently damaged.
There are four types of MS
Relapsing-remitting multiple sclerosis (RRMS)
The most common form of MS. In this type, patients experience MS relapses and periods of stability in between.
Secondary-progressive multiple sclerosis (SPMS) Diagnosed when the problems caused by an exacerbation don’t fully resolve during a remission. This often occurs in patients who were initially diagnosed with RRMS.
Primary-progressive multiple sclerosis (SPMS) Progresses over time, without episodes of remission or improvement of symptoms.
Progressive-relapsing multiple sclerosis (PRMS) It is diagnosed when patients experience escalating symptoms over time, as well as intermittent episodes of remission.
What is the recommended treatment protocol for MS at WORLD HEALTH STEM CELLS?
The World Health Stem Cells recommends the use of hUC-MSCs for the treatment of multiple sclerosis. The specific protocol will be decided by the treating physician after the patient has provided the medical history and undergone the clinical evaluation. The protocol outlined below is just intended to serve as a guide.
- Treatment duration will vary depending on intensity, progression of condition and patient’s health.
- The first day includes a physical examination and blood test.
- Mesenchymal stem cells (MSCs) cultured and expanded from human umbilical cord tissue.
- Injections are given intrathecally and intravenously
- Antioxidant therapy with vitamin C and glutathione with each stem cell treatment
- Ozone therapy with each stem cell treatment
- Platelet-rich plasma therapy with each stem cell treatment
- For more severe cases there may be up to a 3-day course chemo therapy prior to initiating stem cell therapy
What is the treatment protocol for Parkinson’s disease at the WORLD HEALTH STEM CELLS?
The WORLD HEALTH STEM CELLS recommends the use of hUC-MSCs for the treatment of Parkinson’s disease.
For optimal results, we recommend Aggressive Platinum Therapy (APT). APT is a 4-day treatment plan.
Each day you will receive the following:
- Mesenchymal stem cells (MSC) cultured and expanded from human umbilical cord tissue.
- Antioxidant therapy with vitamin C and glutathione
- Ozone therapy
- Platelet-rich plasma therapy (PRP)
Mesenchymal stem cells produce:
- Proteins that help neurons grow and survive
- Angiogenic factors that aid in the healing, growth, development, and maintenance of blood vessels
- Immunomodulatory substances that help to repair the damaged nerves
Researchers are working to develop stem cell treatments restore injured or damaged nerves. For patients with multiple sclerosis, the stem cells repair the damaged areas of demyelination and generate new, healthy cells, preventing further damage and reducing symptoms. The results of preliminary research evaluating the safety and efficacy of autologous stem cell transplantation to treat patients with multiple sclerosis seem promising.
The results of a pilot study conducted by Cleveland Clinic researchers to assess the feasibility, safety, and tolerability of autologous MSC transplantation in MS patients with relapsing-remitting multiple sclerosis (RRMS) or secondary progressive multiple sclerosis (SPMS) were published and found to be feasible, safe, and well tolerated.
Physicians in Barcelona, Spain evaluated the efficacy of mesenchymal stem cells in patients unresponsive to conventional therapy and they too found stem cell transplantation to be both safe and effective. There were fewer enhancing lesions, a reduction in T2 lesion volume, and a reduction in retinal nerve fiber layer (RNFL) thickness after 6 months and 1 year.
Innovative research evaluating the safety and efficacy of stem cell transplantation for the treatment of multiple sclerosis is significantly progressing.
What are the advantages of human umbilical cord mesenchymal stem cells?
- Abundant supply containing up to 10 times more stem cells than bone marrow or adipose derived stem cells
- hUC-MSC have immunosuppressors and immunomodulatory properties that allow their use in any individual without rejection- Human Leukocyte Antigen (HLA) matching is not necessary
- Greater proliferation ability than adult autologous stem cells
They regenerate at a very rapid rate They are young and very adaptive - They have not been impacted by the aging process
- They have not been affected by environmental toxins
- Umbilical cord stem cells can be administered multiple times over the course of days
- Eliminates the need to collect stem cells from the patient’s fat or hip bone reducing pain and recovery time
What are the challenges?
Autoimmune diseases such as MS are difficult to treat because you need your immune system to fight infection, so it is not possible to completely block the patient’s immune system.
- Each patient is different.
- There are many forms of the disease.
- Scientists are still not sure about how the myelin sheath is formed.
UMBILICAL CORD DONATIONS
How Are the Stem Cells Collected?
Our clinic focuses on obtaining healthy stem cells exclusively from umbilical cord blood donors. We collect the placenta once the baby is born, with the parent’s informed consent. Additionally, we follow strict ethical guidelines and collect stem cells from reliable and reputable sources.
INTRAVENUS ADMINISTRATION
How Are the Stem Cells Administered?
Our nursing staff administers the stem cells through an intravenous and intra-pulmonary route. For the most effective outcomes, intravenous administration is preferred.
Are You a Candidate for Stem Cell Therapy?
Stem cell therapy can change the quality of life for many, however not everyone is a candidate. Call our caring, experienced team to find out if stem cell therapy is right for you +1-305-777-7119
Please fill this form
Scientific References:
- Fu et al. Stem cell transplantation therapy in Parkinson’s disease. SpringerPlus (2015) 4:597
- Joyce et al. Mesenchymal stem cell for the treatment of neurodegenerative disease. Regen Med. 2010, November, 5(6)933-946. Doi:10.2217/rme.10.72
- Helena Vilaça-Faria, António J. Salgado and Fábio G.
- Teixeira Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s disease. Cells2019, 8(2), 118; doi:3390/cells8020118
Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev.2015, 11, 288–297. [Google Scholar] [CrossRef] [PubMed] - Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis.2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Med.2010, 5, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.S.; Silva, N.A.; Lourenco, A.S.; Goncalves, V.; Neves, N.M.; Reis, R.L.; Rodrigues, A.J.; Manadas, B.; Sousa, N.; Salgado, A.J. Unveiling the effects of the secretome of mesenchymal progenitors from the umbilical cord in different neuronal cell populations. Biochimie2013, 95, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.A.; Fraga, J.S.; Graos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther.2012, 3, 18. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Salgado, A.J.; Fraga, J.S.; Silva, N.A.; Reis, R.L.; Sousa, N. The secretome of bone marrow mesenchymal stem cells-conditioned media varies with time and drives a distinct effect on mature neurons and glial cells (primary cultures). Tissue Eng. Regen. Med.2011, 5, 668–672. [Google Scholar] [CrossRef]
- Salgado, A.J.; Fraga, J.S.; Mesquita, A.R.; Neves, N.M.; Reis, R.L.; Sousa, N. Role of human umbilical cord mesenchymal progenitors conditioned media in neuronal/glial cell densities, viability, and proliferation. Stem Cells Dev.2010, 19, 1067–1074. [Google Scholar] [CrossRef]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Rep.2017, 7, 4153. [Google Scholar] [CrossRef]
- Serra, S.C.; Costa, J.C.; Assuncao-Silva, R.C.; Teixeira, F.G.; Silva, N.A.; Anjo, S.I.; Manadas, B.; Gimble, J.M.; Behie, L.A.; Salgado, A.J. Influence of passage number on the impact of the secretome of adipose tissue stem cells on neural survival, neurodifferentiation and axonal growth. Biochimie2018, 155, 119–128. [Google Scholar] [CrossRef]
- Assuncao-Silva, R.C.; Mendes-Pinheiro, B.; Patricio, P.; Behie, L.A.; Teixeira, F.G.; Pinto, L.; Salgado, A.J. Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie2018, 155, 83–91. [Google Scholar] [CrossRef] [PubMed]
