CONDITIONS TREATeD / AMYOTROPHIC LATERAL SCLEROSIS (ALS)
Regenerate. Repair. Restore.
Stem Cells Therapy for Amyotrophic Lateral Sclerosis (ALS)

Amyotrophic Lateral Sclerosis (ALS), is a neurodegenerative condition that involves the breakdown of motor neurons in the spinal cord and the brain. Patients with ALS may experience weakness in their limbs followed by a quick and progressive paralysis that leads to total paralysis, respiratory failure and death.
The World Health Stem Cells in Costa Rica, recommends the use of umbilical cord stem cells for the treatment of ALS.
Treatment at World Health Stem Cells could help improve the symptoms of amyotrophic lateral sclerosis:
The treatment could help improve the symptoms of MS:
- Muscle twitches or cramps in the arms, shoulders, legs or tongue (fasciculations)
- Stiff, tight muscles (spasticity)
- Muscle weakness or clumsiness in the hands, arms, legs, ankles, neck or diaphragm
- Slurred speech
- Difficulty swallowing
- Difficulty chewing
- Difficulty walking, tripping, or falling
- Cognitive and behavioral changes
- Difficulty breathing
- Weight loss
What is ALS?
What is ALS?
ALS is a progressive neuromuscular disease that damages primarily the nerve cells in the brain and spinal cord, responsible to controlling voluntary muscle movement like chewing, walking and talking. Damage to the barrier or protective wall between the central nervous system and the blood circulatory system allows harmful substances including immune mediated cells and inflammatory cells to enter both the brain and the spinal cord.
Motor neurons in the brain (upper motor neurons) and motor neurons in spinal cord and motor nuclei of the brain (lower motor neurons) begin to die and no longer send messages to the voluntary muscles in the body. Without signals from the brain and spine the muscles are no longer able to function and they become weak and begin to die. Initially the muscles weaken and begin to twitch but eventually they atrophy or die and the body loses its ability to control voluntary movement.

How can stem cell therapy improve the symptoms of ALS?
A cure for ALS is not available at this time, and the disease typically leads to death within 3–5 years after diagnosis, primarily due to respiratory failure.
Stem cell therapy is a promising approach for the treatment of neurodegenerative disorders such as ALS. Mesenchymal stem cells (MSCs) specifically, seem to be the most suitable type of stem cells due to their demonstrated beneficial effects in many different experimental models, their easy access, and the lack of ethical issues associated with other types of stem cells.
The beneficial effects of mesenchymal stem cells are due to multiple factors. The beneficial effects are due in part to the paracrine effect that results in the release of different growth factors, cytokines, free nucleic acids, lipids and extracellular vesicles. These secreted biomolecules promote tissue repair, modulate the immune system, have an anti-inflammatory effect and provide antiapoptotic activities.1
Human umbilical cord mesenchymal stem cells (hUC-MSCs) can promote the release of acetylcholine, promote neurogenesis and synaptic formation and can reduce oxidative stress and cell death. Research is showing hUC-MSCs to be a better alternative to allogeneic stem cells because of their hypo-immunogenicity, superior tropism, high differentiation potential and paracrine activity.2,3
Evidence suggests HUC-MSCs can differentiate into a variety of neuro-regulatory molecules and can elevate several factors including brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), insulin-like growth factor 1 (IGF-1), Glucagon-like pepetide-1 (GLP-1), and vascular endothelial growth factor (VEGF).4
Neural stem cells transplanted at sites of nerve injury are thought to promote functional recovery by producing trophic factors that induce survival and regeneration of host neurons. Intravenously administered mesenchymal stem cells are also capable of crossing the blood-brain barrier and effectively migrating to regions of neural injury, without inducing tumor growth or an immune response.4.
What are scientists researching?
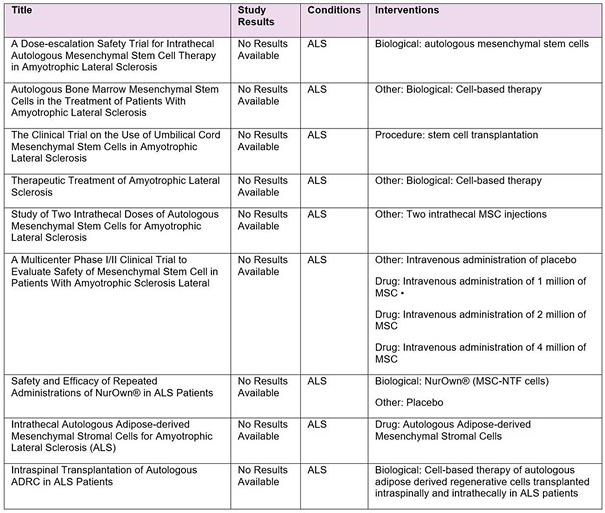
According ClinicalTrials.gov (https://clinicaltrials.gov) on August 22, 2019, there are 9 trials evaluating the safety and efficacy of cell based therapy for the treatment of Amyotrophic Lateral Sclerosis.
Who is at Risk of Developing ALS?
In 2016 the Centers for Disease Control and Prevention estimated that between 14,000 – 15,000 Americans have ALS. ALS usually strikes people between the ages of 40 and 70, and for unknown reasons, military veterans are approximately twice as likely as the general population to be diagnosed with the disease.
Risk Factors:
- Age; Typically striking people between the ages of 40 and 70.
- Gender; Men are at slightly higher risk of developing ALS
- Ethnicity; Caucasians and non-Hispanics are at highest risk
- Military veterans; Twice as likely when compared to the general population to develop ALS
- Genetics; Approximately 5-10 percent of cases are inherited by one parent carrying the genetic mutation
What is the treatment protocol for ALS?
The World Health Stem Cells recommends the use of hUC-MSCs for the treatment of ALS.
For optimal results, we recommend Aggressive Platinum Therapy (APT). APT is a 4-day treatment plan.
Each day you will receive the following:
- Each day the patient receives mesenchymal stem cells (MSC) cultured and expanded from human umbilical cord tissue.
- Antioxidant therapy with vitamin C and glutathione
- Ozone therapy
- Platelet-rich plasma therapy (PRP)
Some studies have found autologous MSCs from patients diagnosed with ALS have reduced properties when compared to MSCs taken from healthy donors. Stem cells from patients with ALS showed reduced expression of several trophic factors and a reduced migration capacity.5-7
NIH trial NCT02881476 showed the intrathecal injection of allogeneic Wharton’s jelly-derived MSCs in ALS patients to be safe.8
What are the advantages of human umbilical cord mesenchymal stem cells?
- Abundant supply containing up to 10 times more stem cells than bone marrow or adipose derived stem cells
- hUC-MSC have immunosuppressors and immunomodulatory properties that allow their use in any individual without rejection- Human Leukocyte Antigen (HLA) matching is not necessary
- Greater proliferation ability than adult autologous stem cells
- They regenerate at a very rapid rate They are young and very adaptive
- They have not been impacted by the aging process
- They have not been affected by environmental toxins
- Umbilical cord stem cells can be administered multiple times over the course of days
- Eliminates the need to collect stem cells from the patient’s fat or hip bone reducing pain and recovery time
What are the challenges?
Autoimmune diseases such as MS are difficult to treat because you need your immune system to fight infection, so it is not possible to completely block the patient’s immune system
- Each patient is different.
- There are many forms of the disease.
- Scientists are still not sure about how the myelin sheath is formed.
UMBILICAL CORD DONATIONS
How Are the Stem Cells Collected?
Our clinic focuses on obtaining healthy stem cells exclusively from umbilical cord blood donors. We collect the placenta once the baby is born, with the parent’s informed consent. Additionally, we follow strict ethical guidelines and collect stem cells from reliable and reputable sources.
INTRAVENUS ADMINISTRATION
How Are the Stem Cells Administered?
Our nursing staff administers the stem cells through an intravenous and intra-pulmonary route. For the most effective outcomes, intravenous administration is preferred.
Are You a Candidate for Stem Cell Therapy?
Stem cell therapy can change the quality of life for many, however not everyone is a candidate. Call our caring, experienced team to find out if stem cell therapy is right for you +1-305-777-7119
Please fill this form
Scientific References:
- Fu et al. Stem cell transplantation therapy in Parkinson’s disease. SpringerPlus (2015) 4:597
- Joyce et al. Mesenchymal stem cell for the treatment of neurodegenerative disease. Regen Med. 2010, November, 5(6)933-946. Doi:10.2217/rme.10.72
- Helena Vilaça-Faria, António J. Salgado and Fábio G.
- Teixeira Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s disease. Cells2019, 8(2), 118; doi:3390/cells8020118
Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev.2015, 11, 288–297. [Google Scholar] [CrossRef] [PubMed] - Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis.2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.; Annett, G.; Wirthlin, L.; Olson, S.; Bauer, G.; Nolta, J.A. Mesenchymal stem cells for the treatment of neurodegenerative disease. Med.2010, 5, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.S.; Silva, N.A.; Lourenco, A.S.; Goncalves, V.; Neves, N.M.; Reis, R.L.; Rodrigues, A.J.; Manadas, B.; Sousa, N.; Salgado, A.J. Unveiling the effects of the secretome of mesenchymal progenitors from the umbilical cord in different neuronal cell populations. Biochimie2013, 95, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.A.; Fraga, J.S.; Graos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther.2012, 3, 18. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Salgado, A.J.; Fraga, J.S.; Silva, N.A.; Reis, R.L.; Sousa, N. The secretome of bone marrow mesenchymal stem cells-conditioned media varies with time and drives a distinct effect on mature neurons and glial cells (primary cultures). Tissue Eng. Regen. Med.2011, 5, 668–672. [Google Scholar] [CrossRef]
- Salgado, A.J.; Fraga, J.S.; Mesquita, A.R.; Neves, N.M.; Reis, R.L.; Sousa, N. Role of human umbilical cord mesenchymal progenitors conditioned media in neuronal/glial cell densities, viability, and proliferation. Stem Cells Dev.2010, 19, 1067–1074. [Google Scholar] [CrossRef]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Rep.2017, 7, 4153. [Google Scholar] [CrossRef]
- Serra, S.C.; Costa, J.C.; Assuncao-Silva, R.C.; Teixeira, F.G.; Silva, N.A.; Anjo, S.I.; Manadas, B.; Gimble, J.M.; Behie, L.A.; Salgado, A.J. Influence of passage number on the impact of the secretome of adipose tissue stem cells on neural survival, neurodifferentiation and axonal growth. Biochimie2018, 155, 119–128. [Google Scholar] [CrossRef]
- Assuncao-Silva, R.C.; Mendes-Pinheiro, B.; Patricio, P.; Behie, L.A.; Teixeira, F.G.; Pinto, L.; Salgado, A.J. Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie2018, 155, 83–91. [Google Scholar] [CrossRef] [PubMed]
